neutrons in selenium|SELENIUM : Baguio Isotopes. Isotopes of an element are atoms that have the same atomic numbers but a different number of neutrons (different mass numbers) in their nuclei. .
Team 7 was a Konohagakure team formed under the leadership of Kakashi Hatake. Two-and-a-half years after Sasuke Uchiha left the village, Kakashi filled out paperwork to form Team Kakashi, with his former pupils Naruto Uzumaki and Sakura Haruno now being treated as equals alongside their teacher. Following Yamato and Sai joining the team, .
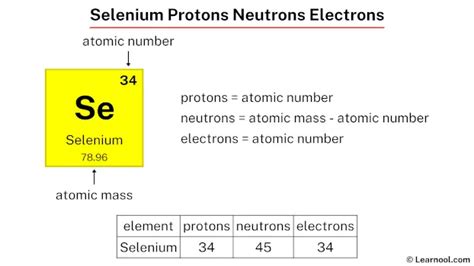
neutrons in selenium,Selenium-76 is composed of 34 protons, 42 neutrons, and 34 electrons. Selenium-77 is composed of 34 protons, 43 neutrons, and 34 electrons. Selenium-78 is composed of 34 protons, 44 neutrons, and 34 electrons. Selenium-79 is composed of .Summary. Atomic Number – Protons, Electrons and Neutrons in Selenium. .
Relative atomic massThe mass of an atom relative to that of carbon-12. This is approximately the sum of the number of protons and neutrons in the nucleus. Where .Neutrons in Selenium. Neutrons are neutral particles found in the nucleus of an atom. The number of neutrons in an atom can vary, and atoms with the same number of protons . Isotopes that have an unstable nucleus and therefore emit particles and energy to reach a more stable balance between protons and neutrons. . Isotopes. Isotopes of an element are atoms that have the same atomic numbers but a different number of neutrons (different mass numbers) in their nuclei. . Selenium Basic Facts. Atomic Number: 34. Symbol: Se. Atomic Weight: 78.96. Discovery: Jöns Jakob Berzelius and Johan Gottlieb Gahn (Sweden) Electron .The number written to the right of the element's name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines .Selenium is a chemical element; it has the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate .Element Selenium (Se), Group 16, Atomic Number 34, p-block, Mass 78.971. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . This is approximately the sum of the number of protons and neutrons in the nucleus. Where more than one isotope exists, the value given is the abundance weighted average.List, data and properties of all known isotopes of Selenium. All atomic nuclei of the chemical element selenium are summarized under selenium isotopes; these all consist of an atomic nucleus with 34 protons and, in the uncharged state, 34 electrons. The difference between each selenium isotope is based on the number of neutrons in the nucleus.Selenium is a Group 5 non-metal element, on the Periodic Table, with an atomic number of 34. About Selenium Selenium has the chemical symbol Se. Molecular Structure Selenium can form many different molecules . Selenium – 75. Se – 75. 34 protons 41 neutrons. Radiation: Decay mode: Electron Caputure. Major Betas: Max E (MeV) Avg E (MeV) # per 100 dis. None. Max.
Name of the isotope: Selenium-75; Se-75 Symbol: 75 Se or 7534 Se Mass number A: 75 (= number of nucleons) Atomic number Z: 34 (= number of protons) Neutrons N: 41 Isotopic mass: 74.92252287 (8) u ( atomic weight of Selenium-75) Nuclide mass: 74.9038718 u (calculated nuclear mass without electrons) Mass excess: -72.16948 MeV Mass defect: .neutrons in selenium SELENIUM Next, find the atomic number which is located above the element 's symbol. Since selenium selenium 's atomic number is 34 34, Se Se has 34 34 protons. 34 34 protons. Fill in the known values where N N represents the number of neutrons. 79 = 34+ N 79 = 34 + N. Rewrite the equation as 34+N = 79 34 + N = 79. 34+N = 79 34 + N = 79.SELENIUM Selenium. It is similar to sulfur in its form and compounds. It converts light into electricity. It decreases electrical resistance with increased illumination. It can convert AC electricity to DC electricity. It is found in several forms, but is usually prepared with a crystalline structure. Most forms are non toxic but hydrogen selenide (H 2 .How many protons and neutrons are there in a selenium-75 atom? Chemical Elements: Each element has a unique atomic number (the number of protons in the nucleus). However, certain elements manifest a phenomenon known as isotopy, whereby the number of neutrons in the nucleus is different. Hence, their mass number (sum of neutrons and .

icon : plus-circle. Protons et neutrons dans le Sélénium. Le sélénium est un élément chimique de numéro atomique 34, ce qui signifie qu’il y a 34 protons dans son noyau. Le nombre total de protons dans le noyau est appelé le numéro atomique de l’atome et reçoit le symbole Z.. La charge électrique totale du noyau est donc +Ze, où e (charge .

Summary. Number of Protons in Selenium. The number of protons can be found by knowing the atomic number of that atom. Number of Protons in Selenium = Atomic number of Selenium = 34. Number of Neutrons in Selenium. The number of neutrons can be found by subtracting the atomic number from its atomic mass. Number .
neutrons in selenium Protons, neutrons and electrons of all elements are mentioned in the table below (You will get the List + Shell diagram of all the elements.) . Selenium has 34 protons, 45 neutrons and 34 electrons: .Caption. Selenium (Se). Diagram of the nuclear composition and electron configuration of an atom of selenium-80 (atomic number: 34), the most common isotope of this element. The nucleus consists of 34 protons .
Neutrons in most abundant isotope: 46: Electron shells: 2,8,18,6 : Electron configuration: [Ar] 3d 10 4s 2 4p 4: Density @ 20 o C: . Selenium is used with bismuth in brasses and as an additive to stainless steel. When .Selenium. It is similar to sulfur in its form and compounds. It converts light into electricity. It decreases electrical resistance with increased illumination. It can convert AC electricity to DC electricity. It is found in several forms, but is usually prepared with a crystalline structure. Most forms are non toxic but hydrogen selenide (H 2 .How many protons, neutrons, and electrons does a selenium atom have with a charge of -2? Atom Composition: Atoms are composed of three basic subatomic particles: protons, neutrons, and electrons. Protons are positively charged particles and are located in the nucleus of an atom along with the neutral neutrons. Electrons have a negative charge .The atomic number of selenium is 34, which means it has 34 electrons. Now it is possible to find the orbital notation of selenium very easily through electron configuration. That is, the orbital notation of selenium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 4. Properties: Selenium has an atomic radius of 117 pm, a melting point of 220.5°C, boiling point of 685°C, with oxidation states of 6, 4, and -2. Selenium is a member of the sulfur group of nonmetallic elements and is similar to this element in terms of its forms and compounds. Selenium exhibits photovoltaic action, where light is converted directly .Name of the isotope: Selenium-80; Se-80 Symbol: 80 Se or 8034 Se Mass number A: 80 (= number of nucleons) Atomic number Z: 34 (= number of protons) Neutrons N: 46 Isotopic mass: 79.916522 (8) u ( atomic weight of Selenium-80) Nuclide mass: 79.8978709 u (calculated nuclear mass without electrons) Mass excess: -77.75926 MeV Mass defect: .
neutrons in selenium|SELENIUM
PH0 · Selenium – Protons – Neutrons – Electrons – Electron Configuration
PH1 · Selenium Facts
PH2 · Selenium
PH3 · SELENIUM
PH4 · Protons, Neutrons, Electrons for Selenium (Se, Se2
PH5 · Number Of Protons, Neutrons, And Electrons In Selenium
PH6 · Chemistry of Selenium (Z=34)
PH7 · CHAPTER 1: The Chemistry of Selenium